“Water Nanostructures Confined inside the Quasi-One-Dimensional Channels of LTL Zeolite”
- Authors
Y. Lee*, C.-C. Kao, S.J. Kim, H.H. Lee, D.R. Lee, T.J. Shin, and J.-Y. Choi
- Journal
Chemistry of Materials
Vol.19, No.25, pp.6252-6257, 2007.12 - DOI
Abstract
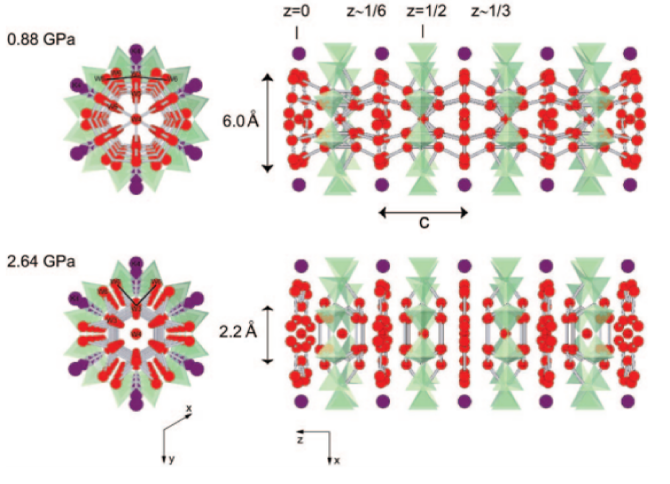
Understanding the formation and evolution of confined water molecules is critical in understanding many chemical and biological processes as well as the water transport inside the Earth. It is often difficult, however, to probe such processes because the host–guest interactions are dynamic in nature. Using a well-defined zeolitic channel as an ideal host and hydrostatic pressure as a driving force, we show how water molecules are introduced and evolve into various confined nanostructures up to 3.37 GPa. In the initial stage of pressure-induced hydration (PIH) occurring inside the undulating 12-ring channels of a synthetic potassium gallosilicate with zeolite LTL topology, water molecules preferentially assemble into hydrogen-bonded clusters, which alternate with water layers. With increasing PIH (by ∼50%) at higher pressures, the interaction between the confined water molecules increases and the water clusters and layers are interconnected to form hydrogen-bonded water nanotubes inside the zeolitic channels. The confined water nanotube closes its maximum access diameter at further increasing pressures and gradually transforms into isolated species interacting with the zeolitic host framework. The evolution of the confined water nanostructures is well-coordinated by the concerted changes in the framework distortion and the re-entrant cation migration, which appear to be driven by the gradual “flattening” of the host 12-ring channels.