“Dehydration-Induced Water Disordering in a Synthetic Potassium Gallosilicate Natrolite”
- Authors
Y. Lee*, S.J Kim, I. Bull, A.J. Celestian, J.B. Parise, C.-C. Kao, T. Vogt
- Journal
Journal of the American Chemical Society
Vol.129, No.44, pp.13744-13748, 2007.10 - DOI
10.1021/ja075037z
Abstract
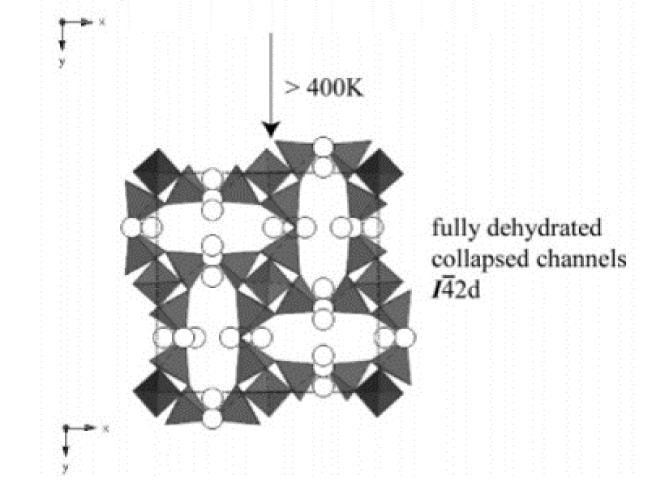
A new potassium gallosilicate zeolite with a natrolite topology (approximate formula K8.2Ga8.2Si11.8O40·11.5H2O) was synthesized under hydrothermal conditions and characterized as a function of temperature using monochromatic synchrotron X-ray powder diffraction and Rietveld analyses. Unlike the previously known tetragonal K8Ga8Si12O40·6H2O phase, the as-synthesized material contains twice the amount of water molecules in an ordered arrangement throughout the channels in an orthorhombic (I212121) symmetry. The ordered configuration of water molecules is stabilized below 300 K, whereas heating above 300 K results in a selective dehydration and subsequent disordering of water molecules in a tetragonal (I4̄2d) symmetry. Above 400 K, the material transforms to a fully dehydrated tetragonal phase with a concomitant volume reduction of ca. 15%. The fully dehydrated material transforms back to its original state when rehydrated over a period of up to 2 weeks. The distribution of potassium cations within the channels remains largely unperturbed during the water rearrangements and their order−disorder transition within the channels.