"Confined water clusters in a synthetic rubidium gallosilicate with zeolite LTL topology"
- Authors
Y. Lee*, S.J. Kim, D-C. Ahn, N-S. Shin
- Journal
Chemistry of Materials
Vol.19, No.9, pp.2277-2282, 2007.05 - DOI
Abstract
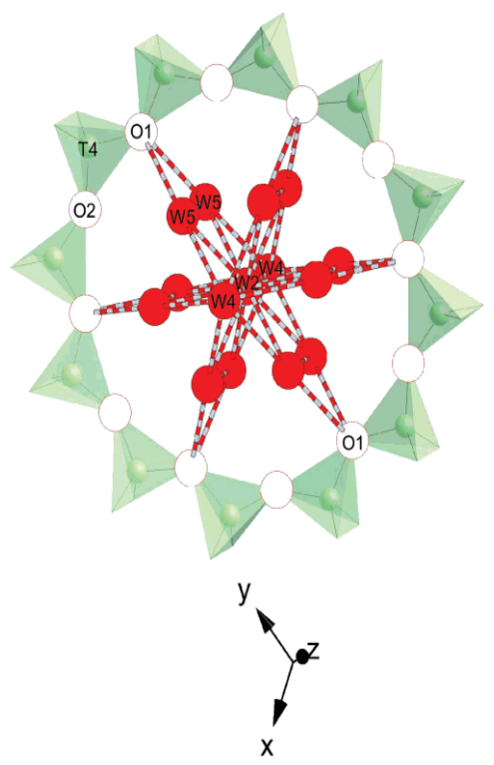
A rubidium gallosilicate with zeolite LTL framework topology has been synthesized under hydrothermal conditions, and the as-synthesized hydrated material was characterized via high-resolution synchrotron X-ray powder diffraction at room temperature. Rb-GaSi-LTL, Rb9.8Ga10.4Si25.6O72·17.5H2O, is hexagonal, space group P6/mmm, with a = 18.6260(1) Å and c = 7.6459(1) Å. The framework model shows complete disordering of Ga and Si in the tetrahedral sites, which is analogous to the framework chemistry of its potassium counterpart, K11Ga10.3Si25.7O72·15.1H2O with a = 18.5525(1) Å and c = 7.5619(1) Å, but contrasts to the proposed preferential aluminum partitioning for the 6-ring sites in the aluminosilicate analogues. Similar to the cation distribution in the potassium analogue, half of the rubidium cations in the unit cell fully occupy the sites associated with the narrow channels on the z = 1/2 plane (sites B and C), whereas the other half fill, on average, five out of the six 8-ring sites in the recessed walls of the main 12-ring channel on the z = 0 plane (site D). All of the water molecules are located along the main 12-ring channels at z = 0, 1/6, 1/3, and 1/2 planes. Water molecules on the latter three planes form clusters within the 12-ring windows confinement via hydrogen bonding to one another and to framework oxygen atoms, while those on the z = 0 plane separate the water clusters by coordinating to the site D cations in the main channel. This partitioning of water molecules in the main channel becomes modulated in the hydrated potassium analogue and seems to be dependent on the chemistry of non-framework cations.