“Pressure-induced structural and electronic changes in alpha-AlH3”
- Authors
J. Graetz, S. Chaudhuri, Y. Lee, T. Vogt, J.T. Muckerman, J.J. Reilly
- Journal
Physical Review B
Vol.74, No.21, pp.214114, 2006.12 - DOI
Abstract
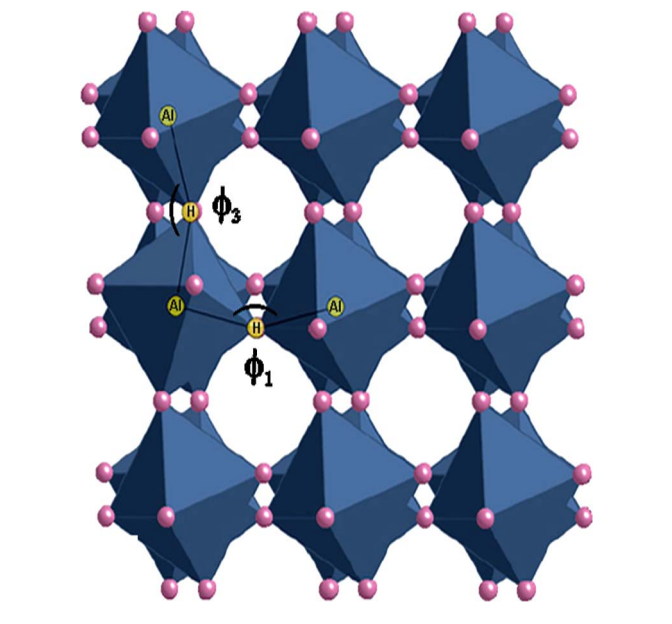
Pressure-induced structural, electronic, and thermodynamic changes in α−AlH3 were investigated using synchrotron x-ray powder diffraction and density-functional theory. No first-order structural transitions were observed up to 7GPa. However, increasing Bragg peak asymmetry with pressure suggests a possible monoclinic distortion at moderate pressures (1–7GPa). The pressure-volume relationship was fit to the Birch-Murnaghan equation of state to give a bulk modulus of approximately 40GPa. The reduced cell volume at high pressure is accommodated by octahedral tilting and a decrease of the Al-H bond distance. Ab initio calculations of the free energy indicate that hydrogenation becomes favorable at H2 pressures above 0.7GPa at 300K. Electronic density of states calculations reveal a slight decrease in the band gap with pressure but no evidence of an insulator-to-metal transition predicted by previous high-pressure studies. Calculated Mulliken charges and bond populations suggest a mixed ionic and covalent Al-H bond at 1atm with an increase in covalent character with pressure.