"High-pressure neutron diffraction study of superhydrated natrolite"
- Authors
M. Colligan, Y. Lee, T. Vogt, A.J. Celestian, J.B. Parise, W.G. Marshall, J.A. Hriljac
- Journal
Journal of Physical Chemistry B
Vol.109, No.39, pp.18223-18225, 2005.10 - DOI
Abstract
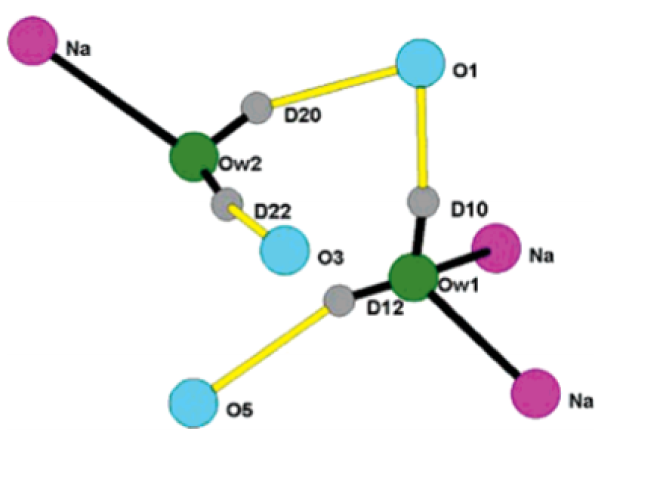
Neutron powder diffraction data were collected on a sample of natrolite and a 1:1 (v/v) mixture of perdeuterated methanol and water at a pressure of 1.87(11) GPa. The natrolite sample was superhydrated, with a water content double that observed at ambient pressure. All of the water deuterium atoms were located and the nature and extent of the hydrogen bonding elucidated for the first time. This has allowed the calculation of bond valence sums for the water oxygen atoms, and from this, it can be deduced that the key energetic factor leading to loss of the additional water molecule upon pressure release is the poor coordination to sodium cations within the pores.