"Variable-temperature structural studies of tetranatrolite from Mt. Saint-Hilaire: Synchrotron X-ray powder diffraction and Rietveld analysis"
- Authors
Y. Lee*, J.A. Hriljac, T. Vogt
- Journal
American Mineralogist
Vol.90, No.1, pp.247-251, 2005.01 - DOI
Abstract
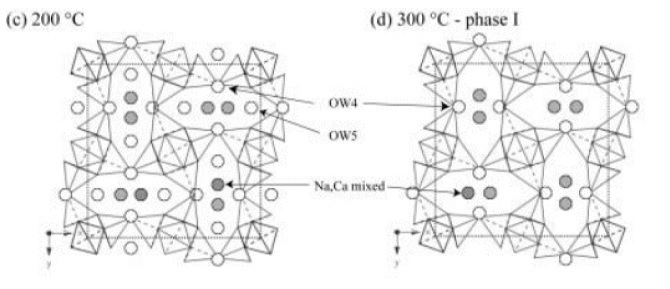
The temperature-dependent evolution of the crystal structure of natural tetranatrolite (Mt. Saint- Hilaire, approximate formula Na5.85Ca1.90Al9.25Si10.75O40·11H2O) was investigated using monochromatic synchrotron X-ray powder diffraction and Rietveld analysis. The room-temperature structural model reveals characteristic Al/Si and Na/Ca disordering over the framework tetrahedral and nonframework cation sites, respectively. Water molecules at the OW4 and OW5 sites along the elliptical channels surround the nonframework cations with full and partial occupancies, respectively, similar to what was observed in previous single crystal studies. As the temperature increases up to 300 °C, the partially occupied OW5 site is gradually dehydrated whereas the fully occupied OW4 site and the disordered Na/Ca site remain fully occupied. Upon complete dehydration of the OW5 site at 300 °C, another phase appears with ~1.8% expansion and ~6.7% reduction of the a- and c-axis parameters, respectively, leading to an overall volume reduction of ~3.3%. In this new phase, the Na and Ca atoms migrate to occupy two closely separated sites along the channels, and 80% of the OW4 water is lost with the remaining water molecules occupying a site close to the previously empty OW5 site. The material decomposes upon full dehydration near 400 °C and becomes X-ray amorphous. The temperature-dependent variations of the T-O-T angles and the chain rotation angle are indicative of the framework relaxation occurring during the selective dehydration and subsequent cation-water migration phase transition.