"Pressure-induced stabilization of ordered paranatrolite: A new insight into the paranatrolite controversy"
- Authors
Y. Lee*, J.A. Hriljac, J.B. Parise, T. Vogt
- Journal
American Mineralogist
Vol.90, No.1, pp.252-257, 2005.01 - DOI
Abstract
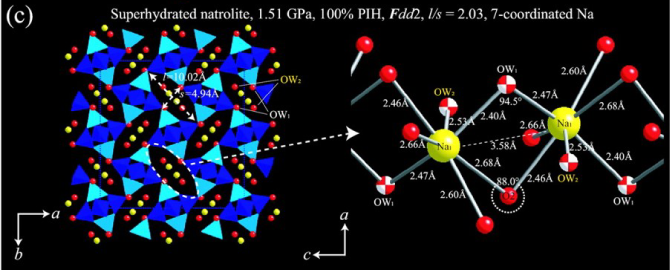
The origin and stability of paranatrolite (approximate formula Na16-xCaxAl16+xSi24-xO80·24H2O), a naturally occurring zeolite with the natrolite topology, has long been debated, with its detailed structure unknown. When taken from an aqueous environment and exposed to the atmosphere, paranatrolite is reported to irreversibly lose water and transform to gonnardite/tetranatrolite, Na16-xCaxAl16+xSi24-xO80·nH2O. Since the latter has a disordered Al/Si distribution over the framework tetrahedral sites, it is believed the same is true for paranatrolite. Natrolite itself (Na16Al16Si24O80·16H2O) has Al/Si ordering, and as recently shown, undergoes a reversible volume expansion (~2.5%) due to pressure-induced hydration (PIH) above 1.2 GPa to a superhydrated phase (Na16Al16Si24O80·32H2O). During this process, an intermediate phase with an even larger volume expansion of ~7.0% has been detected in a narrow pressure range near 1.0 GPa. We report here that this intermediate phase has a unit-cell compatible with the one reported for paranatrolite at ambient conditions with the same 24 water molecules per formula unit and propose that it is paranatrolite with an ordered Al/Si distribution. An unusual watersodium chain is observed in the ordered paranatrolite structure: a sevenfold coordination of sodium cations provided by alternating two water bridges along the expanded elliptical channels. The density of the ordered paranatrolite is lower than those of the 16 and 32 water phases, with its channel openings far more circular than in the low- and high-pressure analogs. The atomistic details of the ordered paranatrolite provide a structural model for the naturally occurring paranatrolite and a complete understanding of this intriguing pressure-volume-hydration mechanism in natrolite, demonstrating the unique role of pressure in controlling the chemistry of microporous materials.