"Pressure-induced migration of zeolitic water in laumontite"
- Authors
Y. Lee, J.A. Hriljac, T. Vogt
- Journal
Physics and Chemistry of Minerals
Vol.31, No.7, pp.421-428, 2004.09 - DOI
Abstract
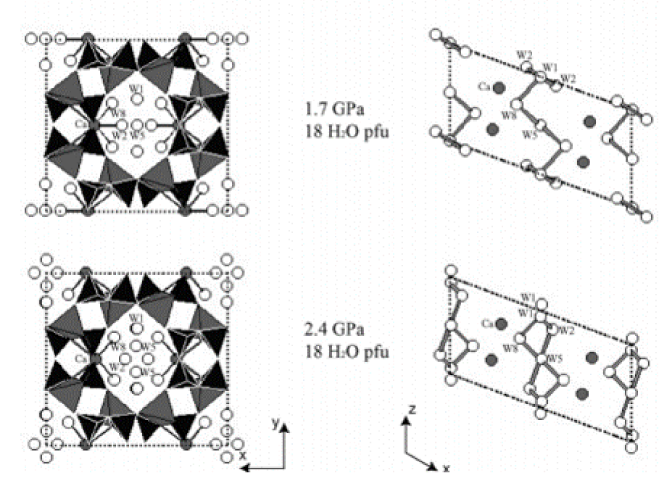
A polycrystalline sample of natural laumontite (Pleasant Valley, Connecticut) was studied up to 6.8 (1) GPa at room temperature using monochromatic synchrotron X-ray powder diffraction and a diamond-anvil cell. A methanol: ethanol: water mixture was used as a penetrating pressure-transmitting fluid. A dry sample measured before adding the pressure fluid inside the diamond-anvil cell contained ~12 H2O per formula unit, consistent with the water content of partially dehydrated laumontite. Upon increasing the pressure to 0.2 (1) GPa, fully hydrated laumontite with 18 H2O per unit cell formed and the unit-cell volume expanded by 2.6%. Further pressure increase up to 2.4 (1) GPa resulted in a gradual contraction of the unit-cell volume and individual cell lengths. During this process, a successive order–disorder transition of hydrogen-bonded water molecules from their symmetry sites was observed, concomittent with an inflectional behavior of the monoclinic beta angle and the channel ellipticity. Above 3 GPa, a tripling of the b axis was detected. Thereafter, up to 6.8 (1) GPa, the compression behavior was reversed for the c axis length and the monoclinic beta angle which showed a gradual increase and decrease, respectively, without any apparent volume discontinuity. We suspect that different ordering of the water molecules or Ca cations inside the channels along the b axis may be responsible for the observed supercell transition above 3 GPa.