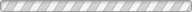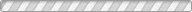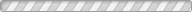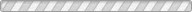"Pressure-induced cation migration and volume expansion in the defect pyrochlores ANbWO(6) (A = NH4+, Rb+, H+, K+)"
- Authors
P.W. Barnes, P.M. Woodward, Y. Lee, T. Vogt, J.A. Hriljac
- Journal
Journal of the American Chemical Society
Vol.125, No.15, pp.4572-4579, 2003.04 - DOI
Abstract
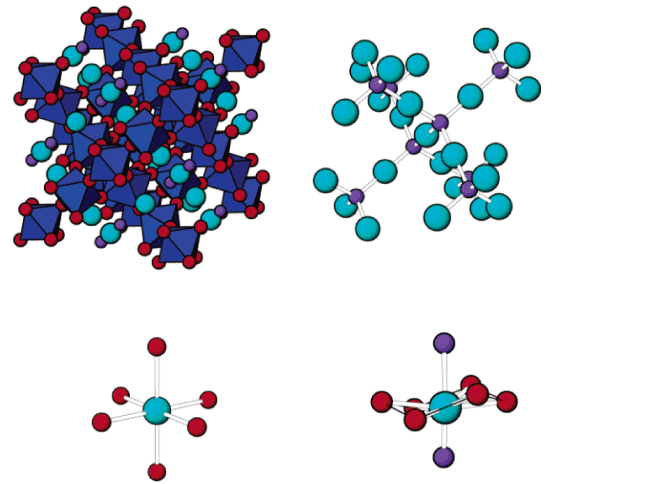
The structural and compositional evolution of four members of the ANbWO6 (A = NH4+, Rb+, H+, K+) defect pyrochlore family have been studied as a function of pressure up to 7 GPa, using a diamond anvil cell and monochromatic synchrotron X-ray powder diffraction. In response to increasing hydrostatic pressure, NH4NbWO6 and RbNbWO6 both initially contract but then undergo a fairly abrupt increase in their unit cell volumes above a characteristic threshold pressure. NH4NbWO6 exhibits a 5.8% increase in the cubic unit cell edge once the pressure exceeds ∼3.4 GPa, while the RbNbWO6 unit cell expansion is larger (∼7.5%) but less abrupt, beginning near 3.0 GPa. Rietveld refinements reveal that the reversible expansion is driven by insertion of water into the structural channels that interpenetrate the NbWO6- octahedral corner sharing framework. The insertion of extra water is accompanied by displacement of the NH4+ or Rb+ ions to a smaller site in the channel structure, which triggers the pressure-induced expansion of the pyrochlore framework. This mechanism explains the counterintuitive expansion of the pyrochlore framework in response to application of external pressure. It should be noted that the expansion exhibited by the pyrochlore framework must coincide with a decrease in the volume of the hydrostatic fluid so that the net volume of the system decreases with increasing pressure. Similar behavior is not observed for KNbWO6·H2O or HNbWO6·H2O, both of which contract in response to increasing pressure. For these smaller monovalent cations, pressure-induced volume expansion does not occur because the hydrated state and subsequent cation shift are already stable at ambient conditions.