"Non-framework cation migration and irreversible pressure-induced hydration in a zeolite"
- Authors
Y. Lee, T. Vogt, J.A. Hriljac, J.B. Parise, J.C. Hanson, S.J. Kim
- Journal
Nature, Vol.4220
No.6915, pp.485-489, 2002.12 - DOI
Abstract
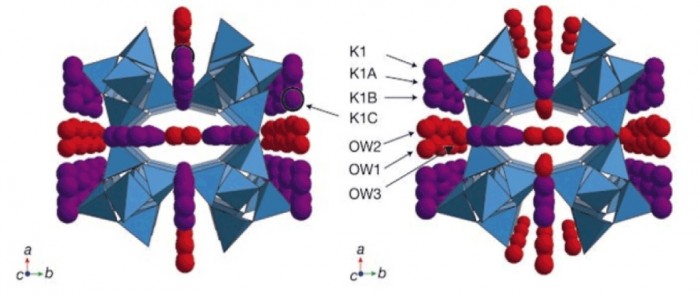
Zeolites crystallize in a variety of three-dimensional structures in which oxygen atoms are shared between tetrahedra containing silicon and/or aluminium, thus yielding negatively charged tetrahedral frameworks that enclose cavities and pores of molecular dimensions occupied by charge-balancing metal cations and water molecules1. Cation migration in the pores and changes in water content associated with concomitant relaxation of the framework have been observed in numerous variable-temperature studies2,3,4,5, whereas the effects of hydrostatic pressure on the structure and properties of zeolites are less well explored6,7,8. The zeolite sodium aluminosilicate natrolite was recently shown to undergo a volume expansion at pressures above 1.2 GPa as a result of reversible pressure-induced hydration9; in contrast, a synthetic analogue, potassium gallosilicate natrolite, exhibited irreversible pressure-induced hydration with retention of the high-pressure phase at ambient conditions10. Here we report the structure of the high-pressure recovered phase and contrast it with the high-pressure phase of the sodium aluminosilicate natrolite. Our findings show that the irreversible hydration behaviour is associated with a pronounced rearrangement of the non-framework metal ions, thus emphasizing that they can clearly have an important role in mediating the overall properties of zeolites.