"Structural changes and cation site ordering in Na and K forms of aluminogermanates with the zeolite gismondine topology"
- Authors
A. Tripathi, J.B. Parise, S.J. Kim, Y. Lee, G.M. Johnson, Y.S. Uh
- Journal
Chemistry of Materials
Vol.12, No.12, pp.3760-3769, 2000.12 - DOI
Abstract
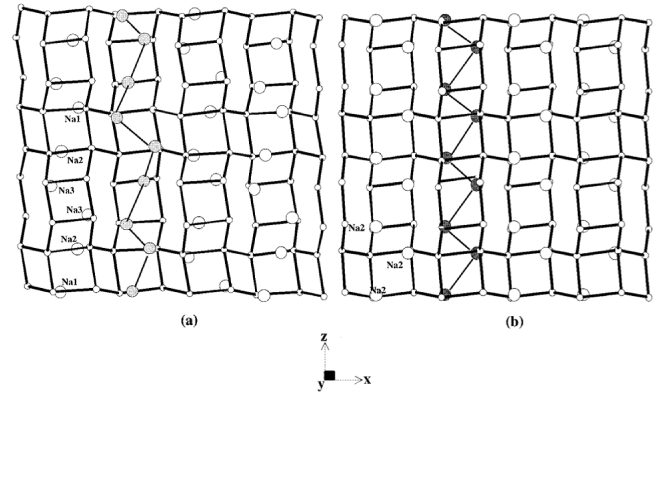
Two new aluminogermanates, K−AlGe-GIS (K8Al8Ge8O32·8H2O) and Na−AlGe-GIS (Na24Al24Ge24O96·40H2O), with the zeolite gismondine (GIS) type framework topology have been synthesized hydrothermally and characterized using single-crystal X-ray diffraction. K−AlGe-GIS crystallizes in the monoclinic space group I2/a with a = 10.311(2) Å, b = 9.749(1) Å, c = 10.225(6) Å, β = 90.000(2)°, and Z = 8. Na−AlGe-GIS crystallizes in the monoclinic space group C2/c with a = 14.490(3) Å, b = 9.940(2) Å, c = 23.530(5) Å, β = 105.90(3)°, and Z = 8. Strict alternation of Ge and Al atoms over the framework sites lowers the symmetry of these aluminogermanates from their topological framework symmetry of I41/amd to the real symmetry I2/a. Both structures consist of twisted double-crankshaft chains running along the a and c axes. In K−AlGe-GIS, potassium and water statistically occupy extraframework sites along the perpendicular eight-ring channels. In Na−AlGe-GIS, all three sodium sites are fully occupied and only three out of seven sites containing water are statistically occupied. A zigzag-ordered arrangement of Na sites in Na−AlGe-GIS results in unit cell symmetry hitherto unobserved for GIS. The synthesis of Na−AlGe-GIS requires the presence of organic bases, while K−AlGe-GIS can be synthesized from gels with or without organic bases. Time-resolved synchrotron X-ray powder diffraction patterns obtained as a function of temperature for Na−AlGe-GIS show a gradual disappearance of the C-centered cell between 150 and 180 °C with a simultaneous appearance of an I-centered monoclinic phase. Calculated powder diffraction patterns indicate that this phase change results from disordering of Na sites in the eight-ring channels. Results of a single-crystal diffraction study for a 50% Na-exchanged K−AlGe-GIS (K4Na4Al8Ge8O32·8H2O) shows that the space group I2/a is retained with a 0.3% increase in the unit cell volume. Both the AlGe frameworks retain the GIS topology until ca. 750 °C after dehydration. The average Al−O−Ge (T−O−T) bond angle of 135.75° in K−AlGe-GIS and 135.29° in Na−AlGe-GIS is smaller than the average Al−O−Si bond angle of 145° in aluminosilicates.