"Synthesis and crystal structures of gallium and germanium variants of cancrinite"
- Authors
Y. Lee, J.B. Parise, A. Tripathi, S.J. Kim, T. Vogt
- Journal
Microporous and Mesoporous Materials
Vol.39, No.3, pp.445-455, 2000.10 - DOI
Abstract
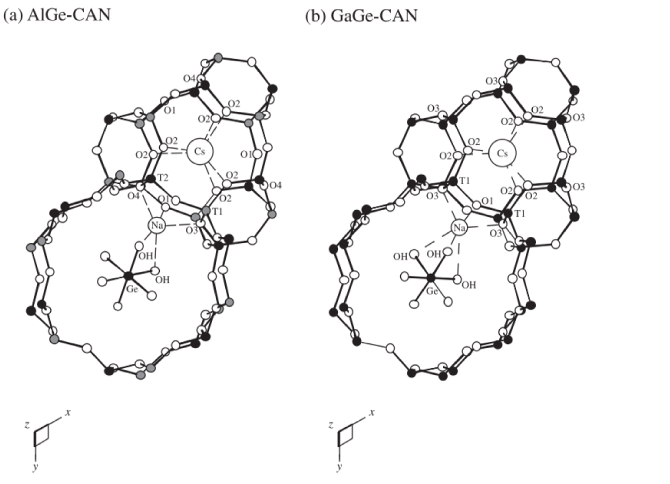
A synthetic aluminogermanate and a gallogermanate with the Cancrinite group (CAN) framework topology have been synthesized under hydrothermal conditions and characterized by single crystal synchrotron X-ray diffraction. AlGe-CAN, Na6Cs2Al6Ge6O24 · Ge(OH)6, is hexagonal, with the space group P63 and a=12.968(1),c=5.132(1) Å, V=747.4(1) Å3. The T-sites exhibit complete ordering of Al and Ge atoms, similar to the framework models of aluminosilicate analogues. GaGe-CAN, Na6Cs2Ga6Ge6O24 · Ge(OH)6, is hexagonal, apparently with the space group P63mc and a=12.950(2),c=5.117(1) Å, V=743.2(2) Å3. Although the observed data are consistent with the presence of the c-glide and consequent disordering of Ga and Ge atoms at the T-sites, calculation using a DLS-optimized framework in the space group P63 reveals that the intensities of the hh2hl reflections with l=2n+1 are less than 0.07% of the strongest (0 0 0 2) reflection, suggesting that P63 is probably the true space group. Resonant diffraction studies performed in the vicinity of the Ga K-edge confirmed the presence of the hh2hl reflections with l=2n+1 and thus confirmed the ordering of the framework Ga/Ge atoms in GaGe-CAN. Inspection of the framework T–O–T bond angles demonstrates greater relative cell contraction for GaGe-CAN compared to AlGe-CAN and aluminosilicate counterparts. In both the structural models, Ge(OH)6 octahedra are occluded in the 12-ring channels running along the 63-axes. The sodium cations fully occupy the sites above the 6-ring windows in the 12-ring channels. The cesium cations fully occupy the sites in the middle of the cancrinite cages. Subtle differences in the coordination geometries of the extra-framework species are found, perhaps due to the pseudo-symmetry of GaGe-CAN. Thermogravimetry results indicate net weight losses of 3.5% and 3.0% for AlGe-CAN and GaGe-CAN, respectively, which are explainable by the dehydration of the Ge(OH)6 octahedra. In situ synchrotron X-ray powder diffraction demonstrated the formation of GaGe analogue of the nepheline hydrate I type structure at the temperature of complete dehydration.