"Structural and Sr2+ ion exchange studies of gallosilicate TsG-1"
- Authors
Y. Lee, S.J. Kim, M.A.A. Schoonen, J.B. Parise
- Journal
Chemistry of Materials
Vol.12, No.6, pp.1597-1603, 2000.06 - DOI
Abstract
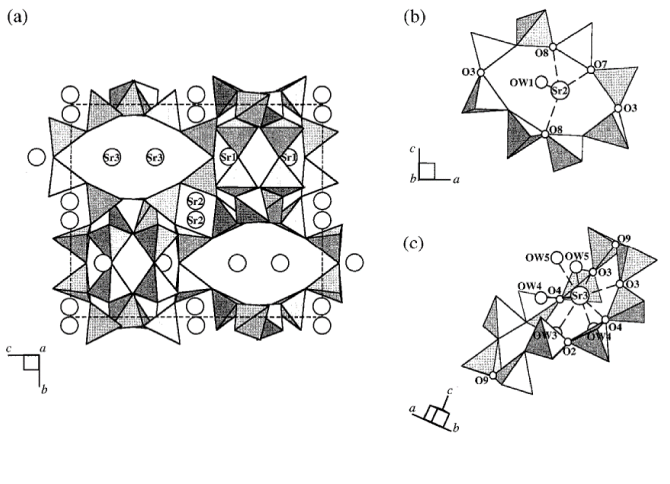
The potassium gallosilicate TsG-1, K10Ga10Si22O64·5H2O, when contacted with a Sr2+:Na+ = 1:5 solution, exhibited a preference for Sr2+ as determined from ion-exchange studies using ion chromatography. A structural model for the strontium-exchanged material, Sr-TsG-1, Sr5Ga10Si22O64·19H2O, was obtained using Rietveld analysis and high-resolution synchrotron X-ray powder diffraction data. The space group, Pnma, of the as-synthesized material is retained in Sr-TsG-1, which has a = 8.6826(1), b = 13.8828(1), c = 16.2484(1) Å. The buckled 8-ring site, consisting of interpenetrated double 6-ring (iD6R) arrays in the CGS topology adopted by TsG-1, is half-occupied by Sr2+ to form a distorted tetrahedral coordination with four framework oxygen atoms. The elliptical 8-ring and s-shaped 10-ring, generated by cross-linking the iD6R arrays along the b and a axis, respectively, also provide environments suitable for Sr2+. An in-situ synchrotron X-ray powder diffraction study confirmed b axis contraction upon Sr2+ uptake into K-TsG-1. Changes in the ellipticity of the s-shaped 10-ring (Δ10) and elliptical 8-ring (Δ8) were monitored from Rietveld refinements based on these data. An abrupt increase in Δ10 is commensurate with the b-axis contraction and suggested a site-specific ion-exchange mechanism. Changes in the cation distribution at each site, however, are not resolved due to a lack of contrast between the effective scattering powers and the interatomic distances involving the sites occupied by Sr2+ and K+ ions.