“Ethylene Epoxidation Catalyzed by Ag Nanoparticles on Ag-LSX Zeolites formed by Pressure- and Temperature-Induced Auto-Reduction”
- Authors
D. Kim, Y. Lee, Y. Kim, K. Mingle, J. Lauterbach, D.A. Blom, T. Vogt, Y. Lee*
- Journal
Chemistry - A European Journal
Vol.24, pp.1041-1045, 2018.01 - DOI
Abstract
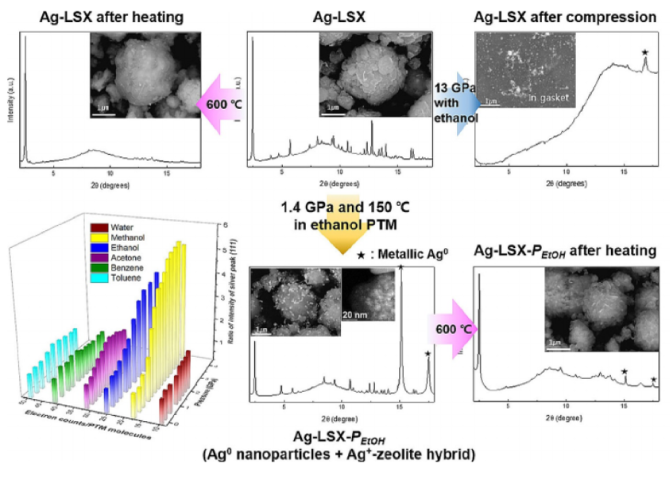
Ag+-Exchanged LSX (Ag-LSX: Ag96Al96Si96O384⋅n H2O), a large pore low silica analogue (Si/Al=1.0) of faujasite, was prepared and post-synthetically modified using pressure and temperature in the presence of various pore-penetrating fluids. Using high-resolution synchrotron X-ray powder and single crystal diffraction we derive structural models of the as-prepared and post-synthetically modified Ag-LSX materials. In the as-prepared Ag-LSX model, we located 96 silver cations and 245 H2O molecules distributed over seven and five distinctive sites, respectively. At 1.4(1) GPa pressure and 150 °C in ethanol the number of silver cations within the pores of Ag-LSX is reduced by ca. 47.4 %, whereas the number of H2O molecules is increased by ca. 40.8 %. The formation of zero-valent silver nanoparticles deposited on Ag-LSX crystallites depends on the fluid present during pressurization. Ag-nanoparticle-Ag-zeolite hybrid materials are recovered after pressure release and shown to have different chemical reactivity when used as catalysts for ethylene epoxidation.