“Dehydration studies of natrolites: Role of monovalent extra-framework cations and degree of hydration”
- Authors
Y. Lee, D. Ahn, T. Vogt, Y. Lee*
- Journal
American Mineralogist
Vol.102, pp.1462-1469, 2017.07 - DOI
Abstract
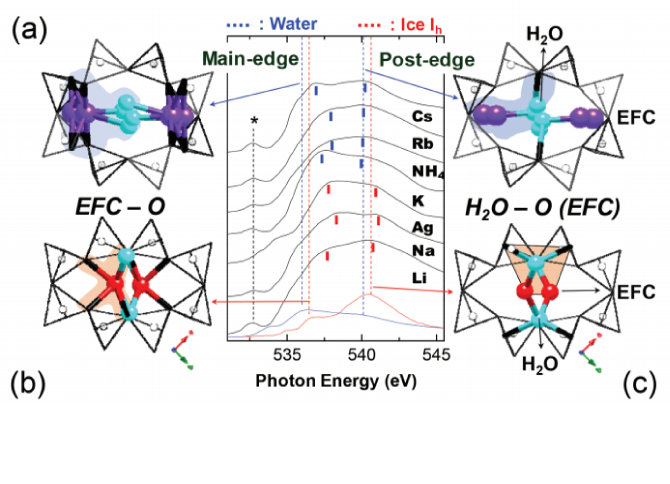
Rietveld refinements of natrolite analogs [M16Al16Si24O80·nH2O, M-NAT, M = Li, Na, Ag, K, NH4, Rb, and Cs, 14.0(1) < n <17.6(9)] at temperatures between 75 and 675 K using synchrotron X-ray powder diffraction reveal the impact H2O content and monovalent extra-framework cations (EFC) contained in the channels have on dehydration and thermal expansion. Dehydration temperatures are found to be inverse proportional to the size of the EFC. Isostructural K-, Rb-, and Cs-NAT with disordered EFC-H2O distribution exhibit negative thermal expansions before dehydration. The thermal expansion coefficients increase linearly from K-, Rb-, to Cs-NAT, the latter exhibits has the smallest thermal expansion coefficient of all NAT analogs [3.0(1) × 10−6 K−1]. After dehydration, the EFC distribution of K-, Rb-, and Cs-NAT becomes ordered and their thermal expansion coefficients become positive. In the isostructural Li-, Na-, and Ag-NAT with ordered EFC-H2O distribution, the thermal expansion coefficients are positive for the Li- and Ag-NAT and negative for Na-NAT. After dehydration, this behavior is reversed, and Li- and Ag-NAT show negative thermal expansion coefficients, whereas Na-NAT exhibits a positive thermal expansion. Upon dehydration, the channels in Li- and Ag-NAT reorient: the rotation angles of the fibrous chain units, ψ, change from 26.4(2)° to –29.6(2)° in Li-NAT and from 22.3(2)° to –23.4(2)° in Ag-NAT. The structure models of the dehydrated Li- and Ag-NAT reveal that the change in the channel orientation is due to the migration of the Li+ and Ag+ cations from the middle of the channel to the walls where they are then coordinated by four framework oxygen atoms. Further heating of these dehydrated phases results in structural collapse and amorphization. X-ray O1s K-edge absorption spectroscopy reveals that the binding energy between the EFC and the oxygen of the framework (Of) is larger in Li- and Ag-NAT than in Cs-NAT due to an increase of the basicity of the framework oxygen. The interaction between the H2O molecules and EFCs allow a clear separation in structures with disordered H2O molecules in the center of the channels (K-, NH4-, Rb-, and Cs-NAT) and those in close proximity to the aluminosilicate framework (Li-, Na-, and Ag-NAT), which leads to systematic dehydration and thermal expansion behaviors. Our structure work indicates that the effects of EFCs are more important to stabilize the NAT structure than the degree of hydration.