“Natrolites with different Fe2+/Fe3+ cation ratios”
- Authors
Y. Lee, T. Vogt, Y. Lee*
- Journal
Microporous and Mesoporous Materials
Vol.244, pp.109-118, 2017.05 - DOI
Abstract
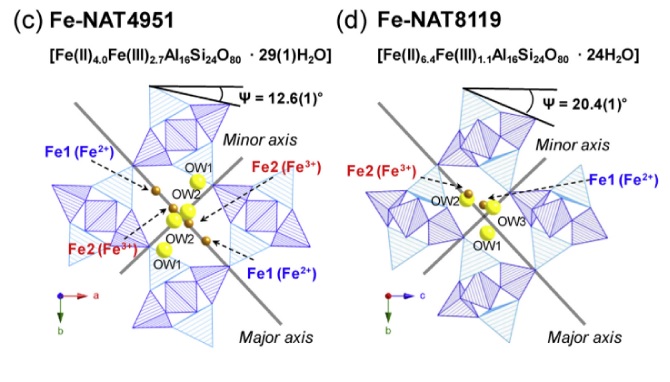
We report the synthesis and structural characterization of two iron-exchanged natrolites, Fe2+4.0Fe3+2.7Al16Si24O80·29(1)H2O (Fe-NAT4951) and Fe2+6.4Fe3+1.1Al16Si24O80·24H2O (Fe-NAT8119) at different pressures and temperatures using ambient, high-temperature and in-situ high-pressure synchrotron powder X-ray diffraction, Mössbauer spectroscopy and extended X-ray absorption fine structure (EXAFS). At ambient conditions, Fe-NAT4951 crystallizes in an orthorhombic structure with space group Fdd2 whereas the structure of Fe-NAT8119 is monoclinic with Cc symmetry. Due to the presence of more H2O molecules in Fe-NAT4951 the channels are more circular as indicated by a T5O10 (T = Si,Al) chain rotation angle of 12.6(1)° compared to 20.4(1)° in Fe-NAT8119. The coordination number of the Fe2+ and Fe3+ cations in the channels of Fe-NAT4951 is 3 and 4, whereas Fe-NAT8119 has 7- and 4-fold coordination, respectively. The two materials behave differently under hydrostatic pressures: due to a discontinuous pressure-induced hydration the volume of Fe-NAT8119 expands by 14.1(1) % near 1.0(1) GPa, whereas the volume of Fe-NAT4951 gradually decreases with pressure. Under increasing temperature and as a result of abrupt dehydration, the unit cell volume of Fe-NAT4951 contracts by ca. 8.3(1) % near 125(1) °C whereas Fe-NAT8119 contracts only by ca. 5.0(1) % near 225(5) °C.