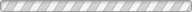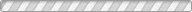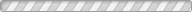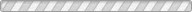“Temperature- and pressure-dependent structural transformation of methane hydrates in salt environments”
- Authors
D. Shin, M. Cha, Y. Yang, S. Choi, Y. Woo, J.-W. Lee, D. Ahn, J. Im, Y. Lee, O.H. Han, J.-H. Yoon
- Journal
Geophysical Research Letters
Vol.10.1002/2016GL072277, pp.2129-2137, 2017.03 - DOI
Abstract
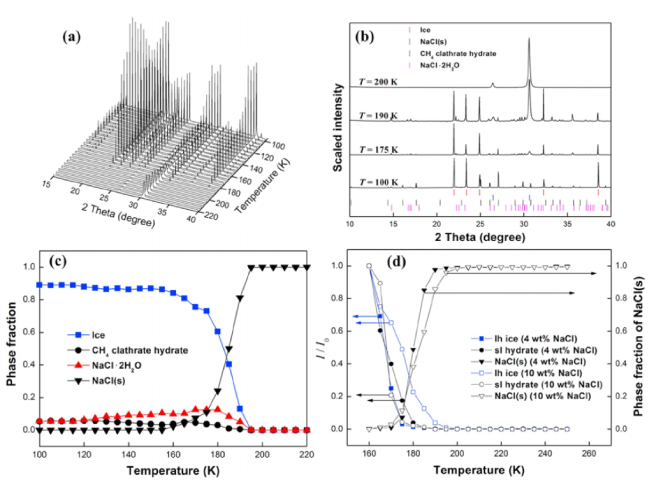
Understanding the stability of volatile species and their compounds under various surface and subsurface conditions is of great importance in gaining insights into the formation and evolution of planetary and satellite bodies. We report the experimental results of the temperature- and pressure-dependent structural transformation of methane hydrates in salt environments using in situ synchrotron X-ray powder diffraction, solid-state nuclear magnetic resonance, and Raman spectroscopy. We find that under pressurized and concentrated brine solutions methane hydrate forms a mixture of type I clathrate hydrate, ice, and hydrated salts. Under a low-pressure condition, however, the methane hydrates are decomposed through a rapid sublimation of water molecules from the surface of hydrate crystals, while NaCl · 2H2O undergoes a phase transition into a crystal growth of NaCl via the migration of salt ions. In ambient pressure conditions, the methane hydrate is fully decomposed in brine solutions at temperatures above 252 K, the eutectic point of NaCl · 2H2O.