“High-Pressure Chemistry of a Zeolitic Imidazolate Framework Compound in the Presence of Different Fluids”
- Authors
“High-Pressure Chemistry of a Zeolitic Imidazolate Framework Compound in the Presence of Different Fluids”
- Journal
Journal of the American Chemical Society
Vol.138, pp.11477-11480, 2016.08 - DOI
Abstract
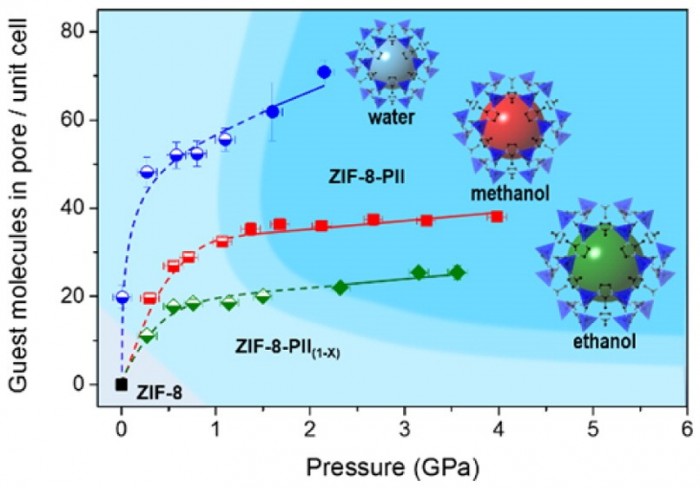
Pressure-dependent structural and chemical changes of the zeolitic imidazolate framework compound ZIF-8 have been investigated using different pressure transmitting media (PTM) up to 4 GPa. The unit cell of ZIF-8 expands and contracts under hydrostatic pressure depending on the solvent molecules used as PTM. When pressurized in water up to 2.2(1) GPa, the unit cell of ZIF-8 reveals a gradual contraction. In contrast, when alcohols are used as PTM, the ZIF-8 unit cell volume initially expands by 1.2% up to 0.3(1) GPa in methanol, and by 1.7% up to 0.6(1) GPa in ethanol. Further pressure increase then leads to a discontinuous second volume expansion by 1.9% at 1.4(1) GPa in methanol and by 0.3% at 2.3(1) GPa in ethanol. The continuous uptake of molecules under pressure, modeled by the residual electron density derived from Rietveld refinements of X-ray powder diffraction, reveals a saturation pressure near 2 GPa. In non-penetrating PTM (silicone oil), ZIF-8 becomes amorphous at 0.9(1) GPa. The structural changes observed in the ZIF-8-PTM system under pressure point to distinct molecular interactions within the pores.