“High-pressure and high-temperature transformation of Pb(II)-natrolite to Pb(II)-lawsonite”
- Authors
J. Im, Y. Lee, D.A. Blom, T. Vogt and Y. Lee*
- Journal
Dalton Transactions
Vol/45, pp.1622-1630, 2016.01 - DOI
Abstract
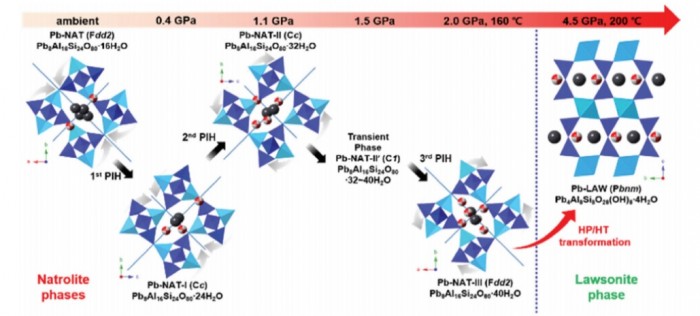
We report on high-pressure and high-temperature chemical transformations of Pb2+-exchanged natrolite (Pb-NAT, Pb8Al16Si24O80·16H2O) using a combination of in situ synchrotron X-ray powder diffraction and ex situ HAADF-STEM real space imaging. Three high-pressure polymorphs of natrolites (Pb-NAT-I, II, III) are observed via step-wise pressure-induced hydrations (PIH) up to 4.5 GPa, during which the number of H2O molecules located inside the natrolite channel increases from 16 to 40 H2O per unit-cell. At 4.5 GPa after heating the high-pressure Pb-NAT-III phase at 200 °C a reconstructive phase transits into a lawsonite phase (Pb-LAW, Pb4Al8Si8O28(OH)8·4H2O) with an orthorhombic space group Pbnm and a = 5.8216(9), b = 9.114(1) and c = 13.320(1) Å is observed. The structure of the recovered Pb-LAW phase was characterized using Rietveld refinement of the in situ synchrotron X-ray powder diffraction data and HAADF-STEM real space imaging. In the recovered Pb-LAW phase the Pb2+ content is close to 42 wt% and as bond valence approximations reveal the Pb2+ cations are more tightly coordinated to the framework oxygen atoms than originally in the natrolite phase.