“Topotactic and reconstructive changes at high pressure and temperatures from Cs-natrolite to Cs-hexacelsian”
- Authors
H. Hwang, D. Seoung, G.D. Gatta, D.A. Blom, T. Vogt, and Y. Lee*
- Journal
American Mineralogist
Vol.100, pp.1562-1567, 2015.07 - DOI
Abstract
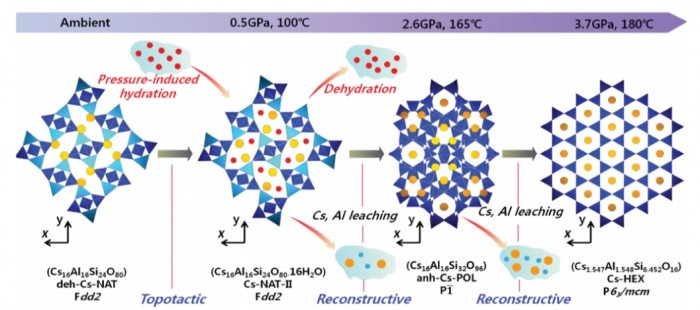
Synchrotron X‑ray powder diffraction experiments have been performed on dehydrated Csexchanged natrolite to systematically investigate successive transitions under high pressures and temperatures. At pressures above 0.5(1) GPa using H2O as a pressure-transmitting medium and after heating to 100 °C, dehydrated Cs16Al16Si24O80 (deh-Cs-NAT) transforms to a hydrated phase Cs16Al16Si24O80·16H2O (Cs-NAT-II), which has a ca. 13.9% larger unit-cell volume. Further compression and heating to 1.5 GPa and 145 °C results in the transformation of Cs-NAT-II to Cs16Al16Si32O96 (anh-Cs-POL), a H2O-free pollucite-like triclinic phase with a 15.6% smaller unit-cell volume per 80 framework oxygen atoms (80Of). At pressures and temperatures of 3.7 GPa and 180 °C, a new phase Cs1.547Al1.548Si6.452O16 (Cs-HEX) with a hexacelsian framework forms, which has a ca. 1.8% smaller unit-cell volume per 80Of . This phase can be recovered after pressure release. The structure of the recovered Cs-HEX has been refined in space group P63/mcm with a = 5.3731(2) Å and c = 16.6834(8) Å, and also been confirmed by HAADF-STEM real space imaging. Similar to the hexacelsian feldspar (i.e., BaAl2Si2O8), Cs-HEX contains Cs+ cations that act as bridges between the upper and lower layers composed of tetrahedra and are hexa-coordinated to the upper and lower 6-membered ring windows. These pressure- and temperature-induced reactions from a zeolite to a feldspar-like material are important constraints for the design of materials for Cs+ immobilization in nuclear waste disposal.