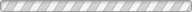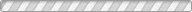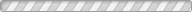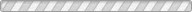“Pressure-Induced Metathesis Reaction To Sequester Cs”
- Authors
J. Im, D. Seoung, S.Y. Lee, D.A. Blom, T. Vogt, C.-C. Kao, and Y. Lee*
- Journal
Environmental Science and Technology
Vol.49, pp.513-519, 2015.01 - DOI
Abstract
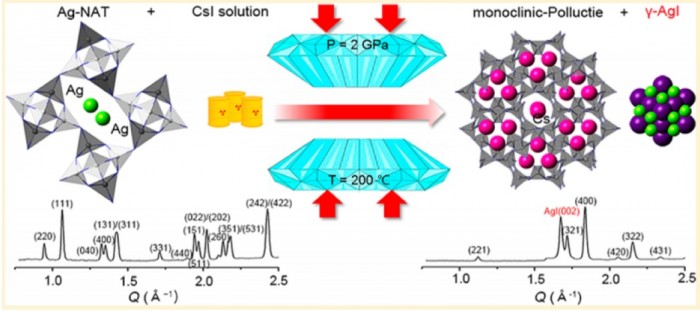
We report here a pressure-driven metathesis reaction where Ag-exchanged natrolite (Ag16Al16Si24O80·16H2O, Ag-NAT) is pressurized in an aqueous CsI solution, resulting in the exchange of Ag+ by Cs+ in the natrolite framework forming Cs16Al16Si24O80·16H2O (Cs-NAT-I) and, above 0.5 GPa, its high-pressure polymorph (Cs-NAT-II). During the initial cation exchange, the precipitation of AgI occurs. Additional pressure and heat at 2 GPa and 160 °C transforms Cs-NAT-II to a pollucite-related, highly dense, and water-free triclinic phase with nominal composition CsAlSi2O6. At ambient temperature after pressure release, the Cs remains sequestered in a now monoclinic pollucite phase at close to 40 wt % and a favorably low Cs leaching rate under back-exchange conditions. This process thus efficiently combines the pressure-driven separation of Cs and I at ambient temperature with the subsequent sequestration of Cs under moderate pressures and temperatures in its preferred waste form suitable for long-term storage at ambient conditions. The zeolite pollucite CsAlSi2O6·H2O has been identified as a potential host material for nuclear waste remediation of anthropogenic 137Cs due to its chemical and thermal stability, low leaching rate, and the large amount of Cs it can contain. The new water-free pollucite phase we characterize during our process will not display radiolysis of water during longterm storage while maintaining the Cs content and low leaching rate.