“Irreversible Xenon Insertion into a Small Pore Zeolite at Moderate Pressures and Temperatures”
- Authors
D. Seoung, Y. Lee, H. Cynn, C. Park, K.-Y. Choi, D.A. Blom, W.J. Evans, C.-C. Kao, T. Vogt, Y. Lee*
- Journal
Nature Chemistry
Vol.6, pp.835-839, 2014.09 - DOI
Abstract
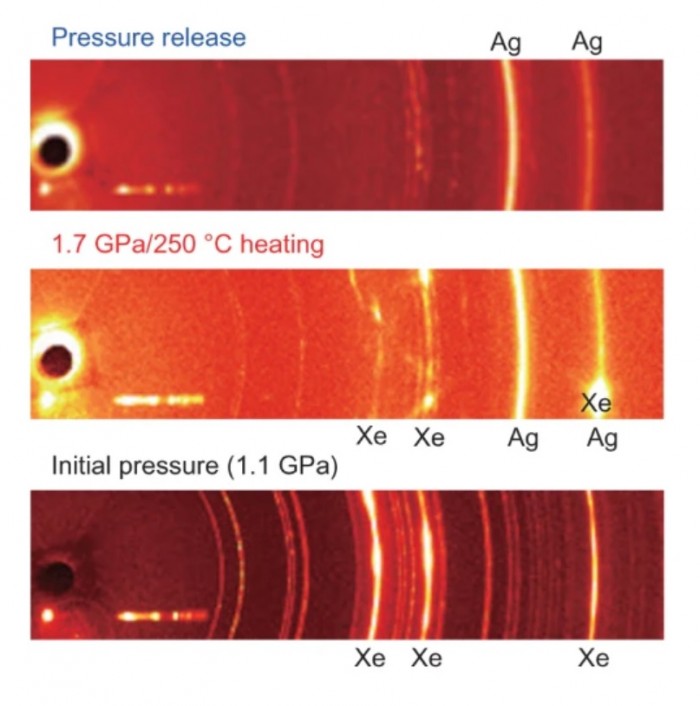
Pressure drastically alters the chemical and physical properties of materials and allows structural phase transitions and chemical reactions to occur that defy much of our understanding gained under ambient conditions. Particularly exciting is the high-pressure chemistry of xenon, which is known to react with hydrogen and ice at high pressures and form stable compounds. Here, we show that Ag16Al16Si24O8·16H2O (Ag-natrolite) irreversibly inserts xenon into its micropores at 1.7 GPa and 250 °C, while Ag+ is reduced to metallic Ag and possibly oxidized to Ag2+. In contrast to krypton, xenon is retained within the pores of this zeolite after pressure release and requires heat to desorb. This irreversible insertion and trapping of xenon in Ag-natrolite under moderate conditions sheds new light on chemical reactions that could account for the xenon deficiency relative to argon observed in terrestrial and Martian atmospheres.