“Uranium(IV) remobilization under sulfate reducing conditions”
- Authors
S.Y. Lee, W.S. Cha, J.-G. Kim, M.H. Baik, E.C. Jung, J.T. Jeong, K. Kim, S.Y. Chung, Y. Lee
- Journal
Chemical Geology
Vol.370, pp.40-48, 2014.02 - DOI
Abstract
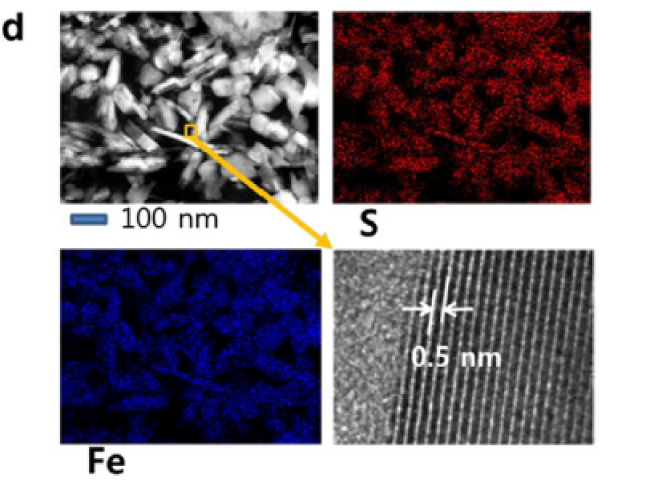
The effects of environmental factors, such as dissolved metal species and their concentrations, on microbial sulfide formation and U(VI) reduction processes are important. During microbial sulfate reduction by Desulfovibrio desulfuricans, the initial aqueous uranium(VI) in our system decreased and later increased as a liquid uranium(IV) suspension. The change in the uranium phase was strongly associated with a biogenic FeS solid (mackinawite), the mineralogical growth of which was affected by the coexisting metal cations (e.g., Ni and Cu). Using laser-induced fluorescence and capillary cell spectroscopy, zeta potentiometry, and ultrafiltration techniques, the produced U(IV) suspension was investigated in situ at a very low level (≤ 10− 6 M) and was identified to be uranium dioxides (UO2) in the form of nm-sized colloids. In addition to the spectroscopic techniques, the direct examination by high-resolution transmission electron microscopy (HRTEM) revealed that the U(IV) phase evidently occurs from the FeS surface in the form of discrete UO2 nanocrystals. Our results indicate that the metal sulfide produced by sulfate-reducing bacteria (SRB) may be an important agent for dispersing the aqueous U(VI) in a colloidal U(IV) form, which would be mobile in porous media such as soils and sediments due to its nanometer-sizes. This study contributes to the understanding of how the FeS metallic conductor abiotically affects the behavior of redox-sensitive uranium by transforming its properties at the mineral surface in sulfate reducing environments.