“Role of Cation-Water Disorder during Cation Exchange in Small-Pore Zeolite Sodium Natrolite”
- Authors
Y. Lee, J.-S. Lee, C.-C. Kao, J.-H. Yoon, T. Vogt, Y. Lee*
- Journal
Journal of Physical Chemistry C
Vol.117(31), pp.16119-16126, 2013.08 - DOI
Abstract
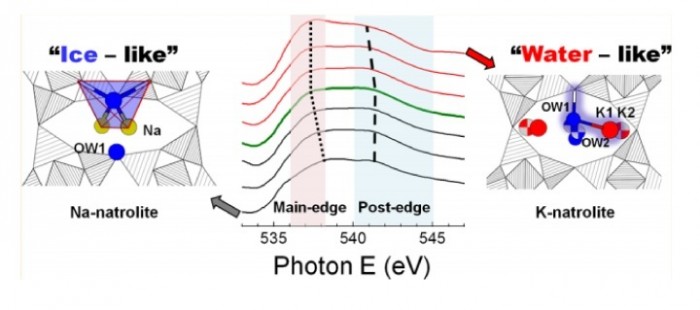
By combining X-ray diffraction with oxygen K-edge absorption spectroscopy we track changes occurring during the K+–Na+ cation exchange of Na-natrolite (Na-NAT) as tightly bonded Na+ cations and H2O molecules convert into a disordered K+–H2O substructure and the unit cell expands by ca. 10% after 50% cation exchange. The coordination of the confined H2O and nonframework cations change from a tetrahedral configuration, similar in ice Ih, with Na+ near the middle of the channels in Na-NAT to two-bonded configuration, similar in bulk water, and K+ located near the walls of the framework in K-NAT. This is related to the enhanced ion-exchange properties of K-NAT, which, in marked contrast to Na-NAT, permits the exchange of K+ by a variety of uni-, di-, and trivalent cations.