“Thermodynamic study of alkali and alkaline-earth cation-exchanged natrolites”
- Authors
L. Wu, A. Navrotsky, Y. Lee, Y. Lee*
- Journal
Microporous and Mesoporous Materials
Vol.167, pp.221-227, 2013.02 - DOI
Abstract
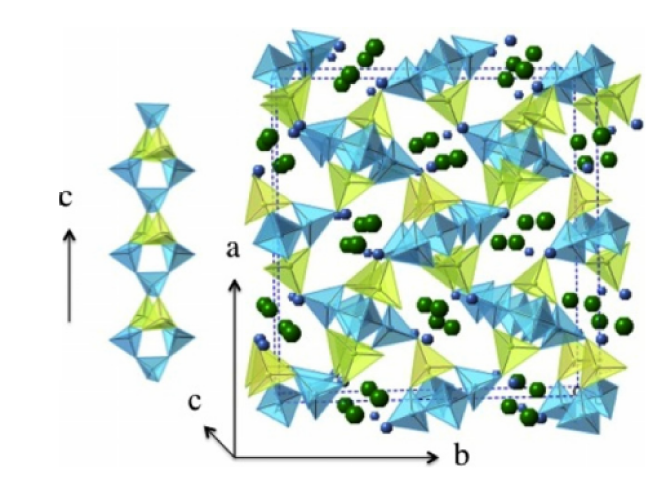
A series of synthetic natrolites with different non-framework cations (Li-NAT, Na-NAT, K-NAT, Rb-NAT, Cs-NAT, Ca-NAT, Sr-NAT and Pb-NAT) are investigated by high temperature oxide melt solution calorimetry. The formation enthalpy from the component oxides (ΔHf,ox) becomes less exothermic with increasing ionic potential (Z/r) of the cations. The dehydration behavior of NATs is examined using TG–DSC. The strength of water binding decreases with increasing cation size. Similar to the trend seen in anhydrous zeolites, a linear dependence of ΔHf,ox on Al/(Al + Si) ratio for hydrous zeolites is established.