“Abiotic reduction of uranium by mackinawite (FeS) biogenerated under sulfate-reducing condition”
- Authors
S.Y. Lee, M.H. Baik, H.-R. Cho, E.C. Jung, J.T. Jeong, J.W. Choi, Y. Lee, Y. Lee
- Journal
Journal of Radioanalytical and Nuclear Chemistry
Vol.296, pp.1311-1319, 2013.02 - DOI
Abstract
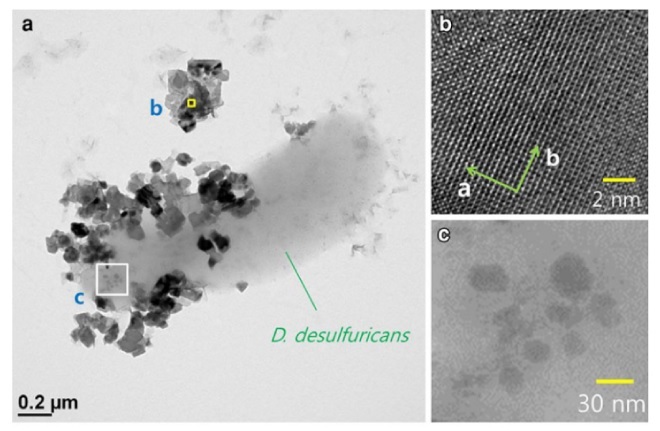
Sulfate-reducing bacteria and their by-products, such as iron sulfides, are widely distributed in groundwater and sediments, and can affect subsurface aqueous chemistry. Here we show the catalytic reduction of hexavalent uranium by FeS particles, which were largely generated by the activities of Desulfovibrio desulfuricans and D. vulgaris in anaerobic condition. Characterization of FeS particles by X-ray diffraction and high-resolution transmission electron microscopy revealed the presence of mackinawite having thin and flexible platy sheets with 0.5-nm lamellar spacing. This biogenic phase mediated abiotic reduction of U(VI) to U(IV) which was confirmed by UV–Vis absorption spectroscopy. The U conversion occurred through surface catalysis that involved adsorption of aqueous U(VI)–carbonate complexes (predominantly UO2(CO3)4−3) onto the mackinawite, but the transformed uranium was then released and remained in suspended form in the solution phase. This surface catalysis and subsequent U(IV) remobilization has not been reported as a pathway to occur under sulfate-reducing conditions. Our results suggest that the iron sulfide solid, which is characteristic of conductive property, is very sensitive and variable depending on the electron supplying and transferring environment, negatively affecting the surface uranium to be strongly stabilized and fixed on the FeS surface.