“Thermal Expansion of the Superhydrated Small-Pore Zeolite Natrolite”
- Authors
Y. Lee*, C.-C. Kao, T. Vogt
- Journal
Journal of Physical Chemistry C
Vol.116, pp.3286-3291, 2012.01 - DOI
Abstract
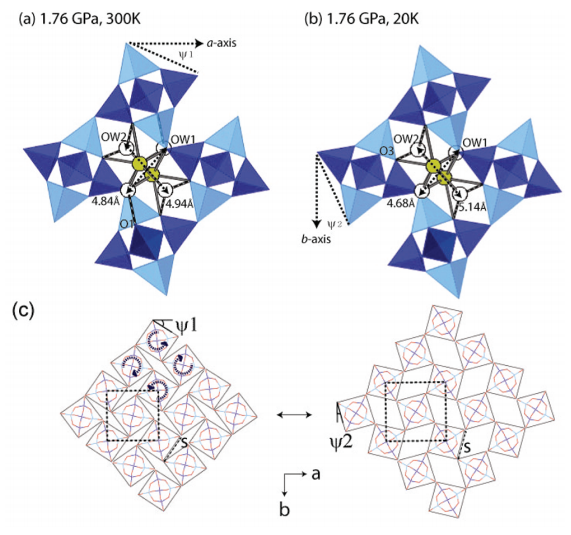
Natrolite (Na16Al16Si24O80·16H2O) at ambient conditions is the paradigmatic example of an auxetic material whose behavior under pressure can be rationalized using a “rotating squares” model with the squares being made up of T5O10 subunits (T = Al, Si). This model also rationalizes reversible superhydration where water is inserted under pressure (“pressure-induced hydration”). Using combined pressure and temperature in situ synchrotron powder diffraction techniques, we have investigated the structural changes occurring in “superhydrated” natrolite (Na16Al16Si24O80·32H2O) between 20 and 300 K at 1.7(3) GPa. Rietveld refinements allowed us to identify significant changes within the sodium–water substructure located in the pores of the superhydrated natrolite. Despite the higher water content of the superhydrated phase, its thermal volumetric expansion coefficient is 15 times larger than that of natrolite at ambient pressure. We put forward a structural descriptor relating thermal expansion and the rotations of T5O10 units that measures the impact of nonframework cations and water contained in the pores on the “rotational squares” mechanism.











