“Sonochemical Synthesis and Properties of Nanoparticles of FeSbO4”
- Authors
P. Nag, S. Banerjee, Y. Lee, A. Bumajdad, Y. Lee, P. Sujatha Devi
- Journal
Inorganic Chemistry
Vol.51, pp.844-850, 2012.01 - DOI
Abstract
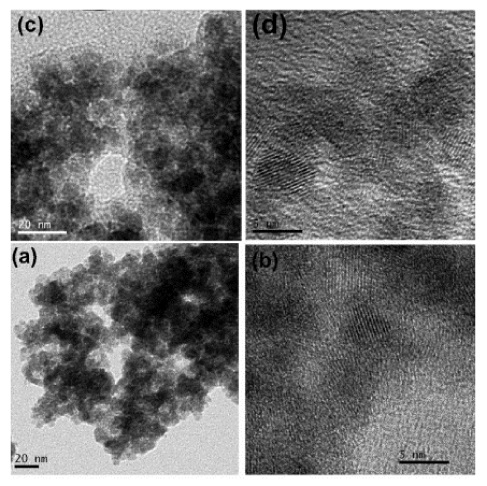
A sonochemical method was employed to prepare reactive nanoparticles of FeSbO4 at 300 °C, which is the lowest calcination temperature reported so far for preparing FeSbO4. A systematic evolution of the FeSbO4 phase formation as a function of temperature was monitored by in situ synchrotron X-ray measurements. The 300 and 450 °C calcined powders exhibited specific surface areas of 116 and 75 m2/g, respectively. The X-ray photoelectron spectra analysis confirmed the presence of mainly Fe3+ and Sb5+ in the calcined powder. The response of the fabricated sensors (using both 300 and 450 °C calcined powders) toward 1000 ppm and 1, 2, 4, and 8% hydrogen, respectively, has been monitored at various operating temperatures. The sensors fabricated using 300 °C calcined powder exhibited a response of 76% toward 4% H2 gas at an operating temperature of 300 °C, while those fabricated using 450 °C calcined powder exhibited a higher response of 91% with a quick recovery toward 4% H2 gas at 300 °C. The results confirmed that a higher calcination temperature was preferred to achieve better sensitivity and selectivity toward hydrogen in comparison to other reducing gases such as butane and methane. The experimental results confirmed that the sonochemical process can be easily used to prepare FeSbO4 nanoparticles for various catalytic applications as demonstrated. Here, we project FeSbO4 as a new class of material exhibiting high sensitivity toward a wide range of hydrogen gas. Such sensors that could detect high concentrations of hydrogen may find application in nuclear reactors where there will be a leakage of hydrogen.











