“Change of Oxidation/Reduction Potential of Solution by Metal-Reducing Bacteria and Roles of Biosynthesized Mackinawite”
- Authors
S.Y. Lee, J.M. Oh, M.H. Baik, Y. Lee
- Journal
Journal of the Korean Mineralogical Society (한국광물학회지)
Vol.24, No.4, pp.279-287, 2011.12 - DOI
Abstract
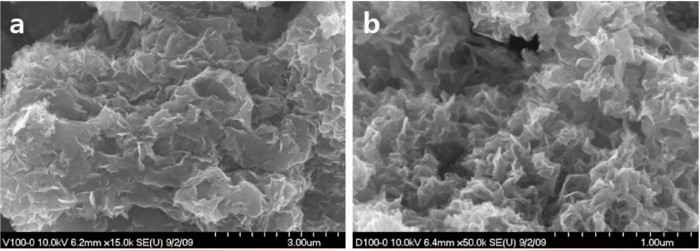
In order to identify if bacteria surviving in soils and groundwater can change the oxidation/reduction potential of groundwater, Eh values of solution that contained bacteria were measured for 2 weeks. The Eh values of the solution reacted with sulfate-reducing bacteria decreased from -120 mV to -500 mV in 5 days, and was superior to in reducing the solution. The Eh value was relatively higher for the solution containing , iron-reducing bacteria, showing -400 mV. During the Eh decrease by the metal-reducing bacteria, a sulfide mineral such as mackinawite (FeS) started precipitating through the microbial reducing process for sulfate and ferric iron. These results show that the ORP of natrual groundwater may be sensitive to the geomicrobial respiration. In addition, a subsurface environment where groundwater is highly reduced and sulfide minerals are largely biogenerated may be a good place to retard the migration of oxidized radionu-clides by making them precipitated as reduced forms.