“Natrolite is not a "soda-stone" anymore: Structural study of alkali (Li(+)), alkaline-earth (Ca(2+), Sr(2+), Ba(2+)) and heavy metal (Cd(2+), Pb(2+), Ag(+)) cation-exchanged natrolites”
- Authors
Y. Lee*, Y. Lee, D. Seoung
- Journal
American Mineralogist
No.96(11-12), pp.1718-1724, 2011.11 - DOI
Abstract
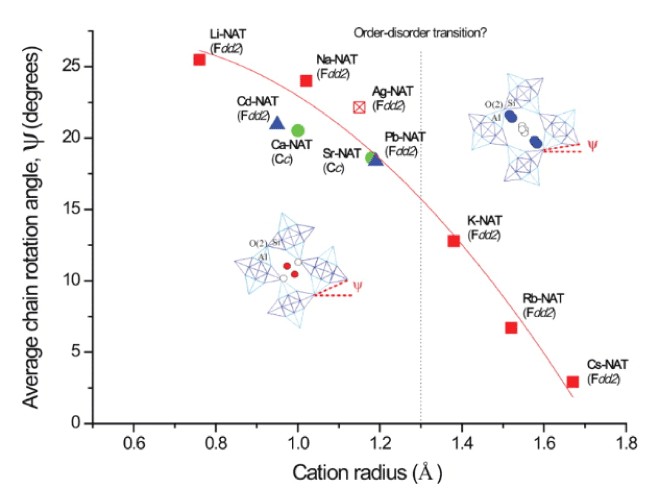
We report here the preparation and structural models of alkaline-earth (Ca2+, Sr2+, Ba2+) and heavy metal (Cd2+, Pb2+, Ag+) cation-exchanged natrolites at ambient conditions and compare them to the alkali (Li+, Na+, K+, Rb+, Cs+) cation forms. The latter two groups all crystallize in the orthorhombic Fdd2 symmetry as the natural sodium natrolite, whereas the alkaline earth analogues are all found in the monoclinic Cc symmetry as scolecite, the natural calcium counterpart. We find the existence of a universal linear relationship between the unit-cell volume and the non-framework cation radius in natrolite. The rotation angles of the fibrous chain units are distributed between 25.5° (Li-form) and 2.9° (Cs-form) to show its inverse proportionality to the non-framework cation radius and the channel opening area. We also propose a possible threshold in the cation radius that dictates the distribution pattern of the non-framework cations and water molecules in the ordered and disordered fashions in natrolite.