“Tetrahedral Atom Ordering in a Zeolite Framework: A Key Factor Affecting Its Physicochemical Properties”
- Authors
J. Shin, D.S. Bhange, M.A. Camblor, Y. Lee, W.J. Kim, I.-S. Nam, S.B. Hong
- Journal
Journal of the American Chemical Society
Vol.133, No.27, pp.10587-10598, 2011.06 - DOI
Abstract
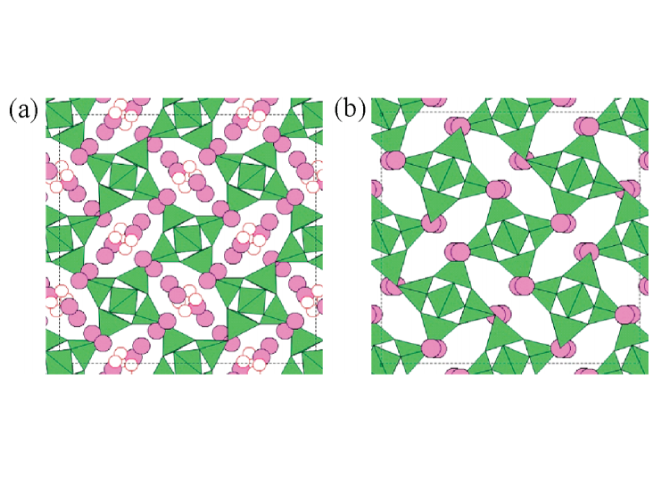
Three gallosilicate natrolites with closely similar chemical composition but differing in the distribution of Si and Ga over crystallographically different tetrahedral sites (T-sites) show striking differences in their cation exchange performance. The ability to exchange Na+ by the larger alkali metal cations decreases upon increasing the size of the cation, as expected, but also with the degree of T-atom ordering. To seek an insight into this phenomenon, the crystal structures of 11 different zeolites, which show variations in degree of T-atom ordering, nature of countercation, and hydration state, have been refined using synchrotron diffraction data. While the three as-made sodium materials were characterized to have a low, medium, and high degree of ordering, respectively, their pore sizes are close to the size of the bare Na+ cation and much smaller than that of the larger alkali cations, which are nonetheless exchanged into the materials, each one at a different level. Interestingly, large differences are also manifested when the Na+ back-exchange is performed on the dehydrated K+ forms, with crystallographic pore sizes too small even to allow the passage of Na+. Although the thermodynamic data point to small differences in the enthalpy of the Na+/K+ exchange in the three materials, comparison of the “static” crystallographic pore sizes and the diameter of the exchanged cations lead us to conclude that during the exchange process these zeolites undergo significant deformations that dynamically open the pores, allowing cation traffic even for Cs+ in the case of the most disordered material. In addition to the very large topological flexibility typical of the natrolite framework, we propose as a hypothesis that there is an additional flexibility mechanism that decreases the rigidity of the natrolite chain itself and is dependent on preferential siting of Si or Ga on crystallographically different T-sites.