“In-situ dehydration studies of fully K-, Rb-, and Cs-exchanged natrolites”
- Authors
Y. Lee, D. Seoung, D. Liu, M.B. Park, S.B. Hong, H. Chen, J. Bai, C.-C. Kao, T. Vogt, Y. Lee*
- Journal
American Mineralogist
Vol.96, No.1, pp.393-401, 2011.03 - DOI
Abstract
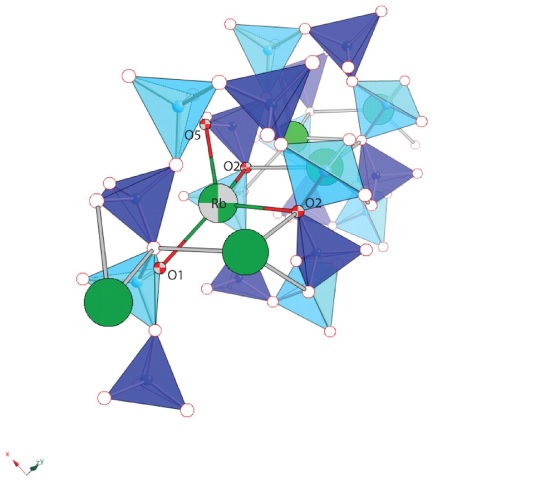
In-situ synchrotron X-ray powder diffraction studies of K-, Rb-, and Cs-exchanged natrolites between room temperature and 425 °C revealed that the dehydrated phases with collapsed frameworks start to form at 175, 150, and 100°C, respectively. The degree of the framework collapse indicated by the unit-cell volume contraction depends on the size of the non-framework cation: K-exchanged natrolite undergoes an 18.8% unit-cell volume contraction when dehydrated at 175 °C, whereas Rband Cs-exchanged natrolites show unit-cell volume contractions of 18.5 and 15.2% at 150 and 100°C, respectively. In the hydrated phases, the dehydration-induced unit-cell volume reduction diminishes as the cation size increases and reveals increasingly a negative slope as smaller cations are substituted into the pores of the natrolite structure. The thermal expansion of the unit-cell volumes of the dehydrated K-, Rb-, and Cs-phases have positive thermal expansion coefficients of 8.80 × 10−5 K−1, 1.03 × 10−4 K−1, and 5.06 × 10−5 K−1, respectively. Rietveld structure refinements of the dehydrated phases at 400 °C reveal that the framework collapses are due to an increase of the chain rotation angles, ψ, which narrow the channels to a more elliptical shape. Compared to their respective hydrated structures at ambient conditions, the dehydrated K-exchanged natrolite at 400°C shows a 2.2-fold increase in ψ, whereas the dehydrated Rb- and Cs-natrolites at 400°C reveal increases of ψ by ca. 3.7 and 7.3 times, respectively. The elliptical channel openings of the dehydrated K-, Rb-, to Cs-phases become larger as the cation size increases. The disordered non-framework cations in the hydrated K-, Rb-, and Csnatrolite order during dehydration and the subsequent framework collapse. The dehydrated phases of Rb- and Cs-natrolite can be stabilized at ambient conditions











