“Gas-Phase Synthesis and Characterization of CH4-Loaded Hydroquinone Clathrates”
- Authors
J.-W. Lee, Y. Lee, S. Takeya, T. Kawamura, Y. Yamamoto, Y.-J. Lee, J.-H. Yoon
- Journal
Journal of Physical Chemistry B
Vol.114, pp.3254-3258, 2010.02 - DOI
Abstract
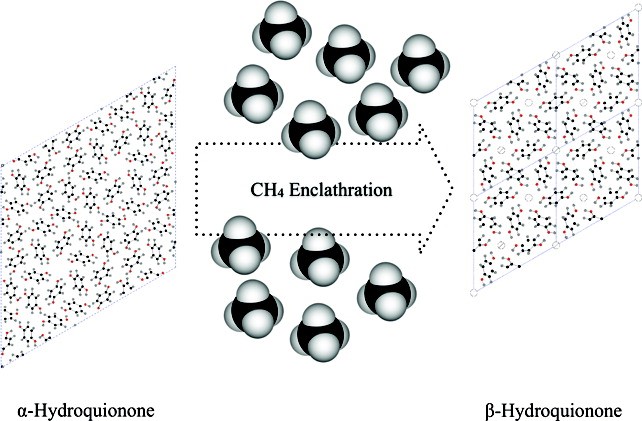
A CH4-loaded hydroquinone (HQ) clathrate was synthesized via a gas-phase reaction using the α-form of crystalline HQ and CH4 gas at 12 MPa and room temperature. Solid-state 13C cross-polarization/magic angle spinning (CP/MAS) NMR and Raman spectroscopic measurements confirm the incorporation of CH4 molecules into the cages of the HQ clathrate framework. The chemical analysis indicates that about 69% of the cages are filled by CH4 molecules, that is, 0.69 CH4 per three HQ molecules. Rietveld refinement using synchrotron X-ray powder diffraction (XRD) data shows that the CH4-loaded HQ clathrate adopts the β-form of HQ clathrate in a hexagonal space group R3 with lattice parameters of a = 16.6191 Å and c = 5.5038 Å. Time-resolved synchrotron XRD and quadrupole mass spectroscopic measurements show that the CH4-loaded HQ clathrate is stable up to 368 K and gradually transforms to the α-form by releasing the confined CH4 gases between 368−378 K. Using solid-state 13C CP/MAS NMR, the reaction kinetics between the α-form HQ and CH4 gas is qualitatively described in terms of the particle size of the crystalline HQ.