“Thermodynamic stability, spectroscopic identification and cage occupation of binary CO2 clathrate hydrates”
- Authors
H.J. Shin, Y.-J. Lee, J.-H. Im, K.W. Han, J.-W. Lee, Y. Lee, J.D. Lee, W.-Y. Jang, J.-H. Yoon
- Journal
Chemical Engineering Science
Vol.64, pp.5125-5130, 2009.08 - DOI
Abstract
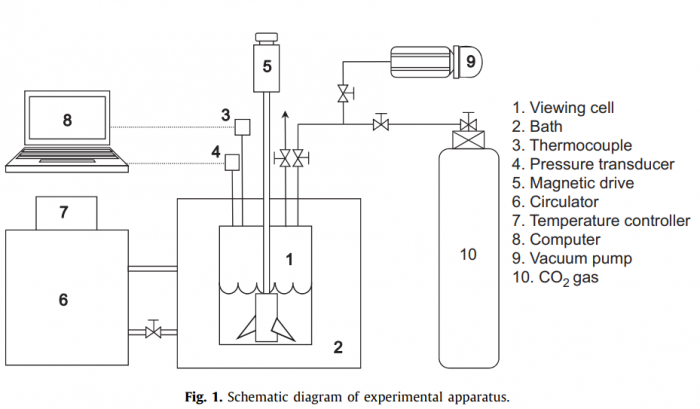
The hydrate phase behavior of CO2/3-methyl-1-butanol (3M1B)/water, CO2/tetrahydrofuran (THF)/water and CO2/1,4-dioxane (DXN)/water was investigated using both a high-pressure equilibrium viewing cell and a kinetic pressure–temperature measurement system with a constant volume. The dissociation pressures of CO2/3M1B/water were identical to those of pure CO2 hydrate, indicating that CO2 is not acting as a help gas for structure H hydrate formation with 3M1B, thus the formed hydrate is pure CO2 structure I hydrate. The CO2 molecules could be encaged in small cages of the structure II hydrate framework formed with both of THF and DXN. For a stoichiometric ratio of 5.56 mol% THF, we found a large shift of dissociation boundary to lower pressures and higher temperatures from the dissociation conditions of pure CO2 hydrate. From the measurements using the kinetic pressure–temperature system, it was found that the solid binary hydrate samples formed from off-stoichiometric THF and DXN aqueous solutions are composed of pure CO2 hydrate with a hydrate number n=7.0 and THF/CO2 and DXN/CO2 binary hydrates with a molar ratio of xCO2·THF·17H2O and xCO2·DXN·17H2O, respectively. The X-ray diffraction was used to identify the binary hydrate structure and Raman spectroscopy was measured to support the phase equilibrium results and to investigate the occupation of CO2 molecules in the cages of the hydrate framework.